Veriva® PPSU is a polyphenylsulfone polymer developed specifically for implantable medical devices that see prolonged or permanent exposure to bodily tissue and fluids. The thermoplastic resin is available in two colors: a transparent grade designated Veriva® V-500 PPSU, and white opaque version designated Veriva® V-501 PPSU.

What are Veriva® PPSU Performance Benefits for Implantable Medical Devices
Veriva® adds biocompatibility for permanent implantation to the inherent physical properties of PPSU, a material with decades of acceptance for short-term medical implant devices. Its attributes include high structural strength, toughness, and compatibility with all common sterilization methods. Additionally, Veriva® PPSU’s transparency and radiolucency facilitate CT, MRI, and x-ray procedures for implanted devices.
What is Veriva®'s Regulatory Compliance for Biocompatibility?
Veriva® PPSU resin is produced by Syensqo* in a dedicated facility that is cGMP-compliant and ISO 13485 certified. The material is tested in ISO 17025 accredited laboratories. Regulatory approvals have been granted in the US, Europe and China. Support for MDMs includes access to the FDA Master File, Global Technical Files and ISO 10993:1 biocompatibility testing data (Table 1).
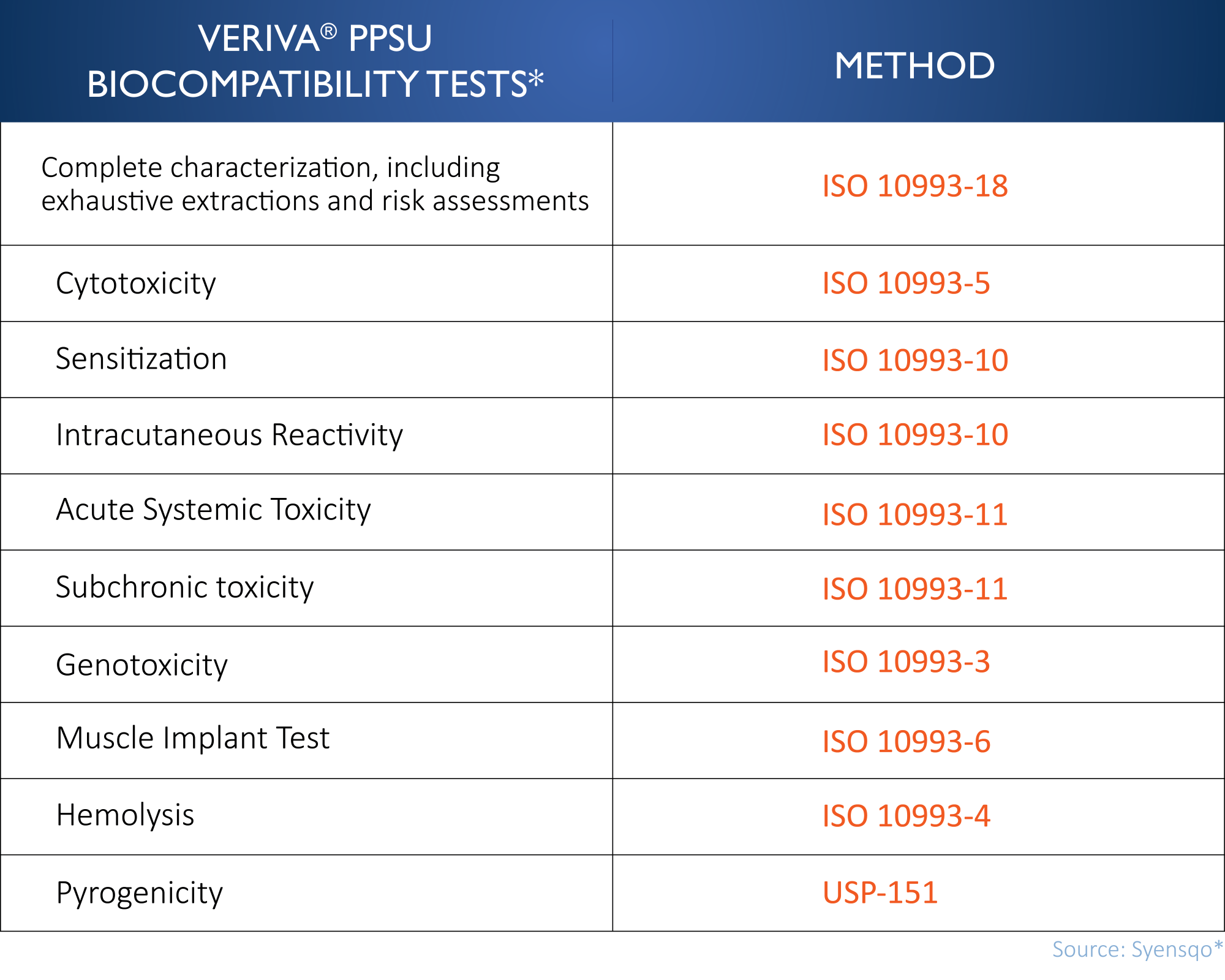
What are Typical Implantable Device Applications for Veriva® PPSU?
Veriva® PPSU can easily withstand the pressures associated with implantable applications such as valves and shunts. This makes the high-performance polymer an excellent candidate for applications such as ventriculoperitoneal, ventriculoarterial and lumboperitoneal shunts for removing excess cerebrospinal fluid (CSF) from the central nervous system for the treatment of hydrocephalus and related conditions affecting the brain.
Genesis Medical Plastics Capabilities in Veriva® Implantable PPSU
Specifications –
SEMI-FINISHED SHAPES
Rod diameters – mm
- 6.4mm – 152.4mm
- Custom & larger diameters on request
Plate thicknesses
- 6.4mm – 31.75mm
- Larger thicknesses on request
- Maximum width 610mm
All semi-finished shapes are made to order, with reasonable minimums.
