With any emerging medical material, the primary concern is safety. The only way to know is to test it, and PEEK, like every biomaterial, has undergone extensive testing to demonstrate its compatibility and safety. These tests have confirmed that PEEK is biocompatible in an array of medical applications, including in vivo applications.
PEEK’s biocompatibility has been demonstrated beyond laboratory testing. The high-performance polymer was first adapted for use as a spinal implant, and that was about 20 years ago.
What does biocompatibility mean?
If a material is biocompatible, it can interface directly with the body’s tissues without causing harm. Few materials fit this description, and those that do may be limited in other ways. Titanium, for instance, is a popular biomaterial for many implants. It is useful for trauma and joint replacement procedures, but it doesn’t flex and bear weight like cortical bone does, which can lead to complications.
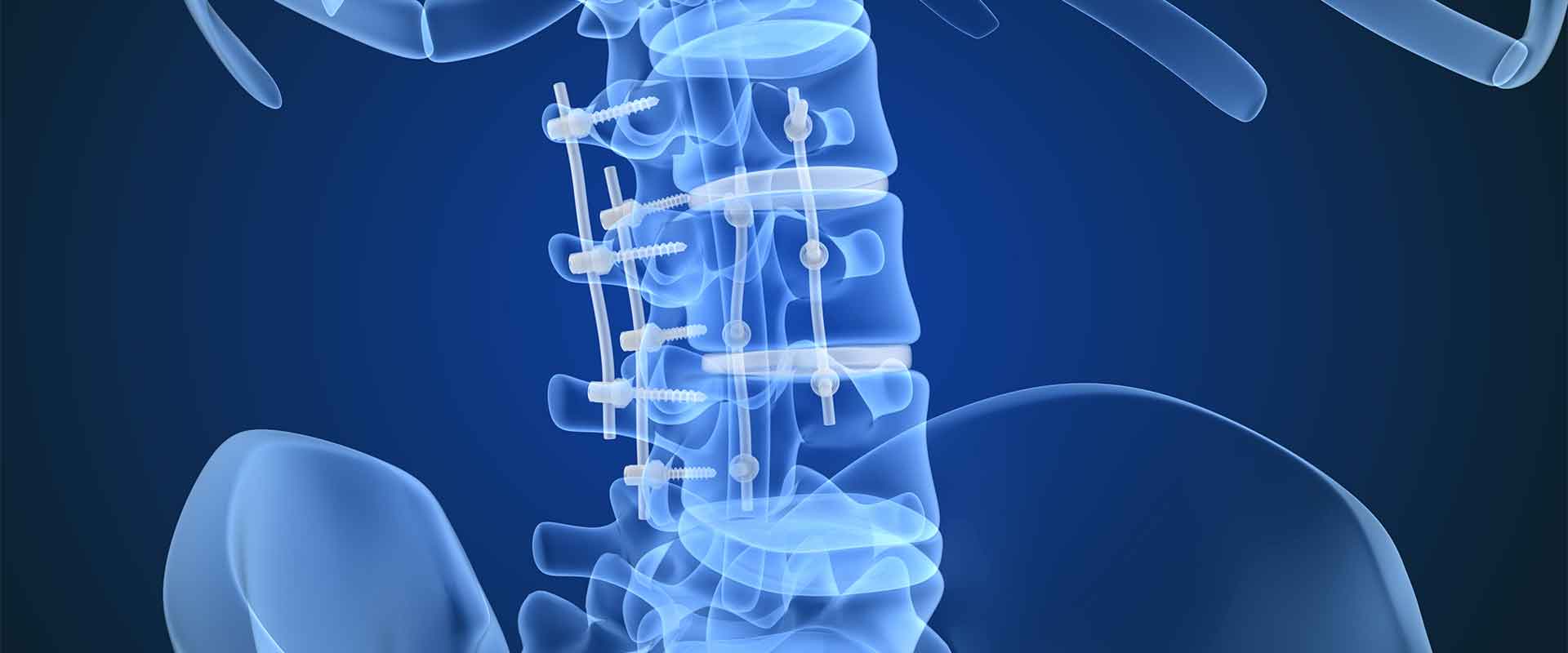
Because PEEK is biocompatible, it can be adapted for many medical applications. PEEK can be injection molded into surgical instruments or laboratory equipment components. The polymer can also be machined into precisely shaped, implantable devices, so whether inside or outside the body, PEEK will not damage the cells it is exposed to. This means PEEK implants can remain in the body indefinitely if needed. Spinal cages, for example, are permanent as they integrate into the fused vertebrae, and PEEK has seen success in this role.
How are medical materials tested for biocompatibility?
Before any potential biomaterial is utilized in medicine, it must undergo the most rigorous testing available. The FDA groups all medical devices into one of three categories, depending on their potential for harm, should the device produce complications. Any implantable device is placed in the Class III category, which is the most aggressively regulated class. Only about 10 percent of all medical devices end up in this category, but because they support or preserve life, they must be handled carefully.
The USP (United States Pharmacopeia) is a respected medical organization that publishes pharmaceutical information and standards, and it is recognized by the U.S. government as a valuable standard. The USP’s Class VI testing is its most demanding and determines whether or not a material is safe for in vivo use. Class VI testing consists of three testing procedures, including:
1. Acute System Toxicity Test – The Acute System Toxicity Test, also called the Systemic Injection Test, considers the material’s irritant potential and capacity for harm. During the test, the material is delivered to an animal subject either orally, dermally or via inhalation. Researchers will select the appropriate delivery method, depending on how the material will be used should it be approved for human applications.
Once the material is delivered to the subject, the animal is monitored for 72 hours. At the end of this period, all subjects are assessed for any reaction to the material. If there are signs of irritation, it is scored so that researchers can standardize their findings.
The Acute System Toxicity Test will reveal any general toxicity to the material. This may take the form of weight loss or a general decline in health.
2. Intracutaneous Test – The Intracutaneous Test is the next step in determining biocompatibility. During this test, the material is placed in direct contact with the tissues it will be expected to interface with. In most versions of the test, the material is injected into 10 total sites, split among two animal subjects. The goal is to see whether the material is harmful to the tissues it will be in contact with.
Like with the Acute System Toxicity Test, the animal subjects are monitored for 72 hours, with assessments at 24, 48 and 72 hours. Researchers will compare all test sites to a control, so if there are any signs of toxicity, it will be readily apparent.
The Intracutaneous Test determines if the material is a local irritant, as opposed to a general, whole-body hazard.
3. Implantation Test – The Implantation Test is the final step before confirming biocompatibility. During this test, the material is implanted in a pair of animal subjects, usually in the subjects’ muscle tissue. The Implantation Test lasts for five or seven days, and following its conclusion, the implant sites are compared to controls, and their condition is assessed.
The Implantation Test is the last stop before a material can be considered biocompatible. It is useful for determining whether a material will provoke a response at the cellular level.
If the material passes Class VI testing, that means it is not cytotoxic (harmful to cells), genotoxic (harmful to the genetic material inside cells) or immunogenic (provokes an immune response).PEEK’s biocompatibility has been demonstrated using this form of testing, and it has undergone many rounds of testing to confirm the findings. Researchers and medical professionals are comfortable with the proof behind PEEK’s impressive safety record.