Eviva™ polysulfone is a tough, rigid polymer for implantable medical devices in contact with bodily tissue and fluids long-term, or periods of 30 days or more. It is available in its natural transparent light yellow tint and opaque white.

Are the Properties of Eviva™ the Same as Traditional Polysulfone?
Eviva™ PSU physical properties are similar to those of standard polysulfone. The resin manufacturer’s investments in processing technology and biocompatibility testing have resulted in a version of PSU that complies with regulatory standards for use in implantable devices in contact with internal tissue and fluids long-term.
What Are the Benefits of Eviva™ for Implantable Medical Devices?
The properties of Eviva™ PSU translate to tough, strong implantable medical devices that do not absorb fluids. Unlike traditional metallic biomaterials, devices made from this dimensionally stable thermoplastic are radiolucent, an advantage for artifact-free medical imaging.
Eviva™ PSU can be converted into intricate devices by injection molding or by machining parts from stock shapes extruded from the polymer. Its process versatility provides more design freedom and production efficiencies compared to manufacturing medical devices from metals.
What is Eviva™ Polysulfone’s Biocompatibility Compliance?
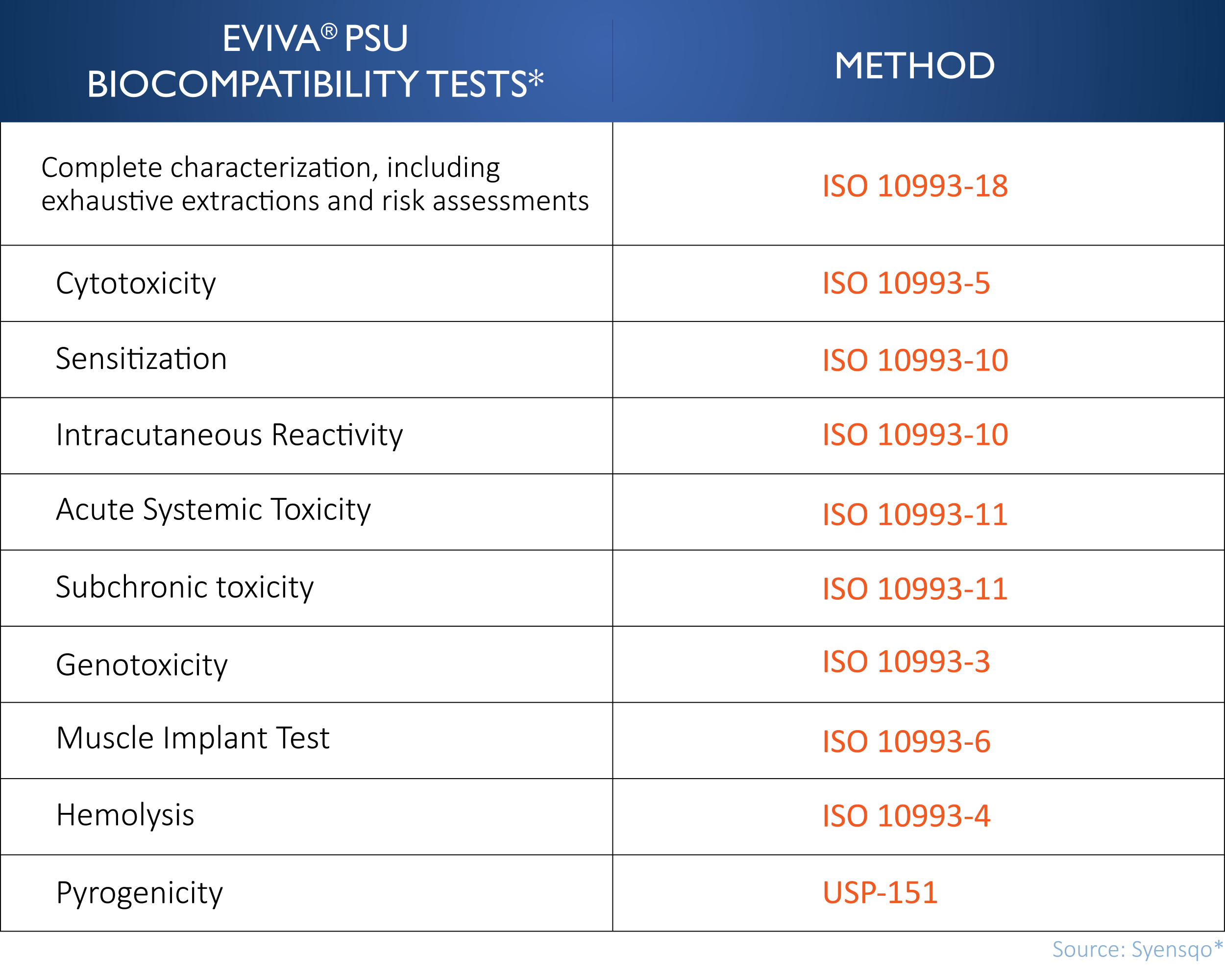
Eviva™ PSU is produced in an ISO 13485 certified and cGMP-compliant facility. It is tested in ISO 17025 accredited laboratories and has regulatory approvals in the US, Europe and China. Syensqo, the resin supplier, supports to MDMs on development projects with access to the FDA Master File, Global Technical Files and ISO 10993:1 biocompatibility testing data (Table 1).
What Are Typical Applications for Eviva™ implantable Polysulfone?
Cardiovascular, neurovascular, drug delivery and dental devices number among Eviva™ PSU’s common, non-load bearing applications. The polymer’s ductility, light weight and transparency make it a viable option for many low-profile components for optimized, low-risk vascular or injection access.
Genesis Medical Plastics Capabilities in Eviva™ Implantable Polysulfone
Specifications –
SEMI-FINISHED SHAPES
Rod diameters – mm
- 4mm – 152.4mm
- Custom & larger diameters on request
Plate thicknesses
- 4mm – 31.75mm
- Larger thicknesses on request
- Maximum width 610mm
All semi-finished shapes are made to order, with reasonable minimums.
