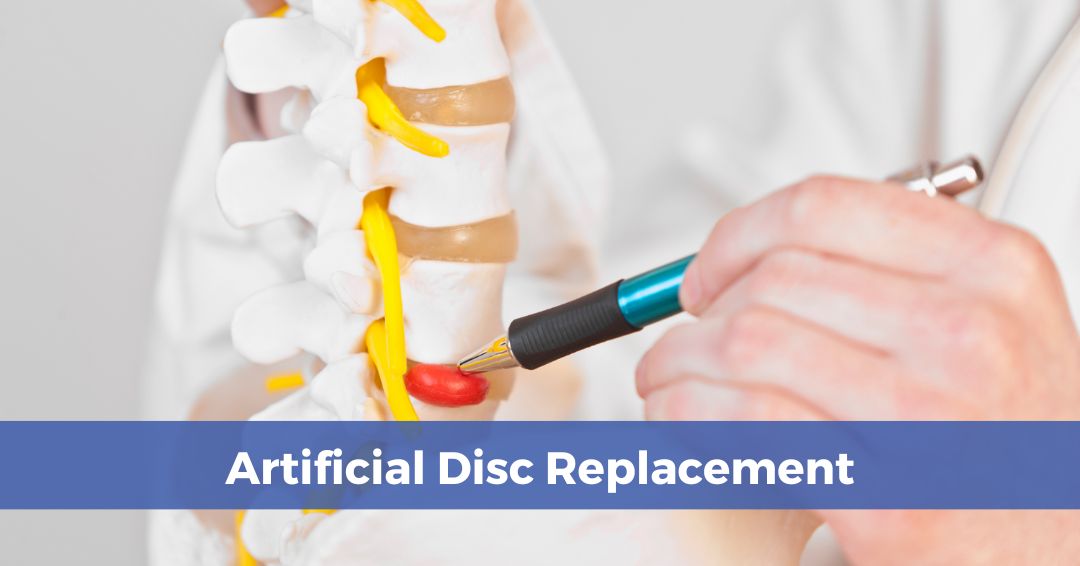
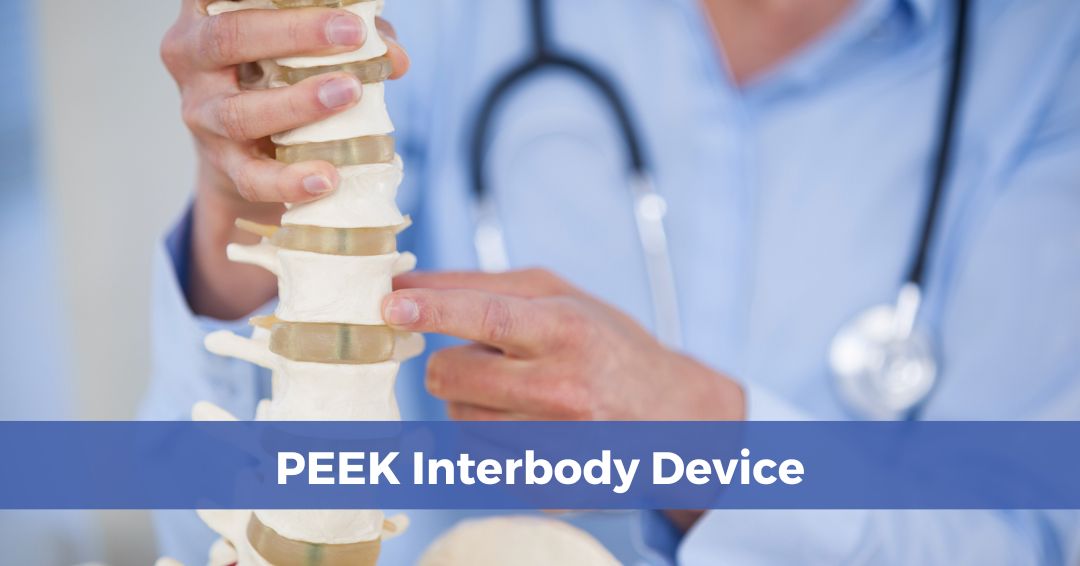
Lumbar cages are spinal implants used in patients suffering from chronic or degenerative back conditions, including:
- Spinal stenosis
- Degenerative disc disease
- Scoliosis
- Spondylolisthesis
- Fractures, tumors or infections
While some people with the above conditions may not experience symptoms, some experience debilitating pain and loss of flexibility. For these patients, a PEEK lumbar cage can provide relief.
Why is PEEK an Ideal Biomaterial for a Lumbar Cage?
PEEK’s first medical application was in spinal fusion, where it has been used as an interbody implant for roughly two decades. PEEK has been featured in most forms of spinal fusion procedures, including anterior cervical discectomy and fusion (ACDF), anterior lumbar interbody fusion (ALIF) and posterior lumbar interbody fusion (PLIF). Since its introduction, the high-performance polymer has rapidly become the first choice in lumbar cages for several reasons, including:
- An ideal flexural modulus – In its unfilled state, PEEK has a flexural modulus that is similar to cortical bone. As such, it isn’t a loadbearing material, but a load-sharing one. Because it behaves like cortical bone, it’s easier for surgical teams to anticipate the implant’s performance and secure a precise fit.More importantly, PEEK’s bone-like modulus means it won’t rob constructive stresses from nearby bone, which results in stress shielding and, potentially, subsidence. A study published in the European Spine Journal confirmed this and showed that subsidence rates associated with titanium implants was at or above 20 percent. By contrast, PEEK spinal implants demonstrated subsidence rates less than 10 percent.This may be why the same European Spine Journal study found that patient outcomes were better with PEEK lumbar cages, compared to titanium cages.
- Pure radiolucency – Radiolucency (transparency to imaging techniques) is valued in fusion cages, as it ensures the implant will not interfere with attempts to image the spine. PEEK’s pure radiolucency is one of its most important features. PEEK is completely invisible on X-rays, CT scans and MRIs, so surgical teams can spot potential complications before they manifest, and assess how the implant is fusing with native bone.In applications where radiolucency is not preferred, the polymer can be mixed with additives including barium sulfate to add in image contrast. The barium sulfate can impart this contrast without compromising the polymer’s properties.
- Total biocompatibility – PEEK has produced excellent outcomes in patients for 20 years, but long before it achieved this success, it was thoroughly tested for biocompatibility. The FDA requires implantable materials to pass through the most demanding safety assessments, and in the U.S., this includes successfully completing multiple tests like ISO 10993 and USP Class VI testing. PEEK is one of the few polymers that has done so, confirming its safety in implant procedures.ISO 10993 is considered the most comprehensive approach to biocompatibility testing and is recognized as such by the FDA. This is why the FDA considers ISO 10993 compliance for premarket approval purposes.The ISO 10993 standard includes 20 sections, and several are relevant to lumbar cage manufacturers. This includes recommended testing procedures for cytotoxicity, system toxicity and dermal sensitization. Risk management is also a priority throughout the process, so implants must be studied for any leachables or extractables.
All testing must be done with a sample representative of the final implant. This means the sample must be converted, processed, packaged and sterilized like an implant intended for the patient. The exact testing procedures are derived from standards produced by other organizations, so they are proven to be effective.
During USP Class VI testing, the biomaterial is introduced to animal tissues. Testing protocols include a systemic injection test, an intracutaneous test and an implantation test. The goal of biocompatibility testing is to verify that the material is not cytotoxic, genotoxic or immunogenic, and these tests confirm it using a variety of tissues. This includes the tissues that will interface with the implant.
- Processability – PEEK’s processability has made it a favored material among engineers, as it can be converted into an incredible array of components. This processability advantage is also relevant in medicine, where PEEK can be machined to extremely tight tolerances. This means a reliable implant, and because PEEK is endlessly processable, it can be sized to fit a patient’s anatomy. Current generation PEEK spinal implants can already be sized up or down to fit different patients, without loss of implant performance.When processed by an experienced PEEK converter, the polymer is also easier to process than metals.
The above advantages have propelled PEEK to frontline status among interbody fusion cages, and with PEEK, the future of lumbar cages is even brighter.
The Present and Future of PEEK Lumbar Cages
Though PEEK has already developed an impressive track record in medicine, it is still a relatively new biomaterial. That means there is plenty of potential left to unlock with the material.
One active area of research is improving osseointegration between implant and native bone, and current generation PEEK spinal implants are already showing the fruits of this research. For example, some PEEK implants are now designed with microporous structures, so the bone is encouraged to lock tightly into the implant and integrate in a stable fashion. Additional materials can also be mixed with PEEK to encourage this bone-in growth as well.
For instance, PEEK lumbar cages augmented with hydroxyapatite or zeolite can encourage bone growth by inhibiting osteoclast activity, which results in bone resorption. Several implants featuring these materials have already been studied and research confirms that they stimulate better osseointegration.
The future of PEEK is bright, but so is the present. With compelling advantages like pure radiolucency, a bone-like modulus, excellent biocompatibility and versatile processing options, PEEK lumbar cages are an excellent choice in the present, and the most promising biomaterial well into the future.