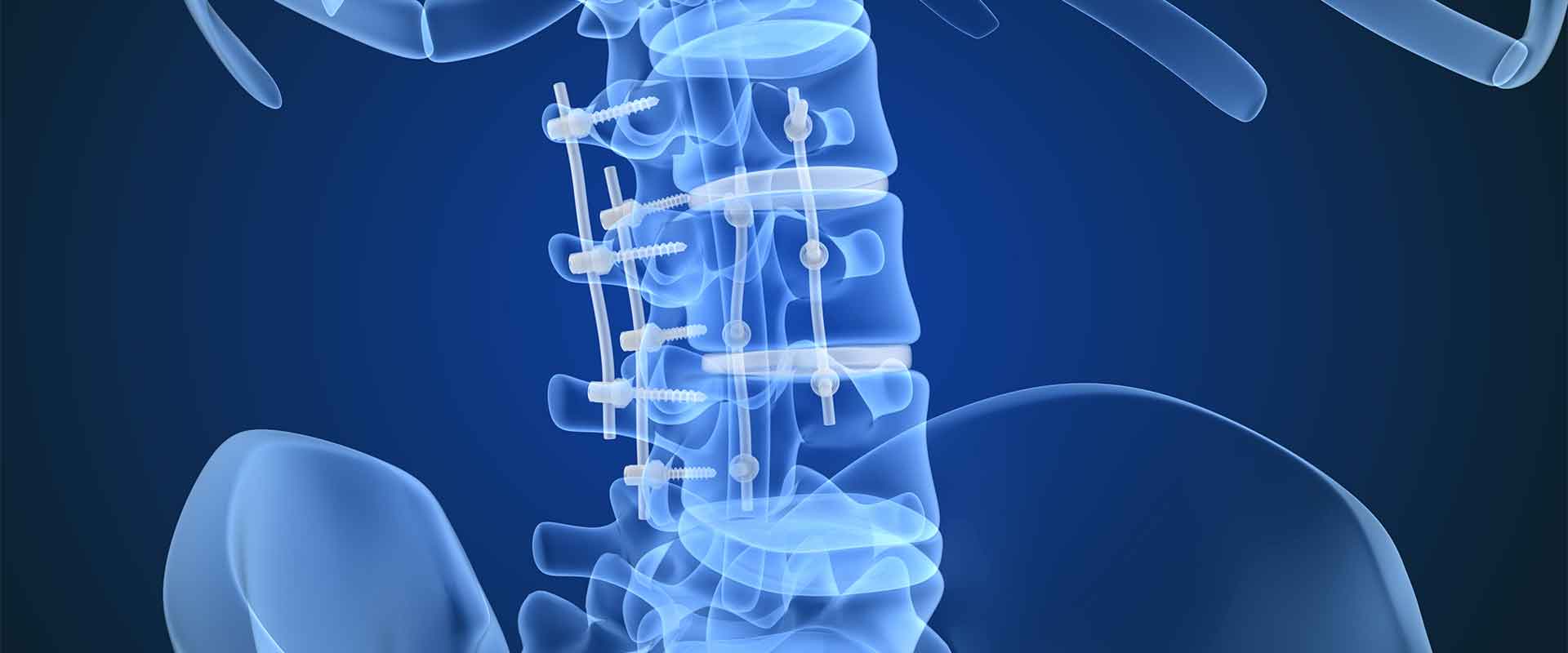
What is a cervical cage and what is it used for?
 A PEEK cervical cage is an interbody spinal implant used during anterior cervical discectomy and fusion (ACDF). It provides a stable surface for bone to fuse to and ensures a secure lock between implant and vertebrae. Most cervical cages are made from high performance biomaterials like PEEK, because the implant is subjected to constant compressive forces, and because the implant is permanent.
A PEEK cervical cage is an interbody spinal implant used during anterior cervical discectomy and fusion (ACDF). It provides a stable surface for bone to fuse to and ensures a secure lock between implant and vertebrae. Most cervical cages are made from high performance biomaterials like PEEK, because the implant is subjected to constant compressive forces, and because the implant is permanent.
Until about 20 years ago, titanium was the primary choice for cervical cages, but the emergence of PEEK has supplanted it. Now, PEEK is the frontline choice and is being improved upon all the time.
Why is PEEK the right choice for cervical interbody implants?
PEEK was introduced to medicine in the form of an interbody cage, used in ACDF procedures. From the beginning, it was clear that PEEK was perfectly suited to this role, for several reasons. Some of them include:
1. A favorable flexural modulus – Interbody fusion cages provide an interface for two vertebrae to fuse together, so ideally, the cage would behave like bone too. That’s what PEEK does because it possesses an similar modulus to cortical bone. PEEK bears weight like bone, flexes and bends like bone and its tension strength is similar to bone’s as well. With these attributes, PEEK behaves predictably once implanted.
What is perhaps more important, though, is that this modulus supports proper bone healing. PEEK is a load sharing material, so it will not rob native bone of necessary, bone-stimulating stresses. That means less stress shielding and less subsidence, which is a noted problem with titanium cages.
If additional stiffness is required, PEEK can be mixed with carbon fiber to provide the required increased stiffness.
2. Pure radiolucency – PEEK’s other sizeable advantage is its pure radiolucency, which means it is completely invisible on X-rays, CT scans and MRIs. This degree of radiolucency is essential for ACDF procedures, as it ensures surgical teams can track how well it is integrating with native bone. If there are emerging complications, PEEK’s radiolucency ensures they can be caught early and mitigated.
PEEK’s radiolucency can be modified, if needed. In its natural, unfilled form, PEEK possesses pure radiolucency. When mixed with additives such as barium sulfate, additional image contrast can be imparted into the polymer. This is usually driven by the type of implantable application and is sometimes used for spinal fusion procedures, but it has higher usage in other implantable application areas.
3. Complete biocompatibility – All implantable biomaterials must pass through the most rigorous safety testing available. In this case, that means the U.S. Pharmacopeia’s (USP) Class VI testing protocols and a number ISO standards, including ISO 10993.
ISO 10993 is the medical device industry’s leading biocompatibility testing standard, and is recognized by the FDA and most European nations as such. The newest version of the standard was published in 2018, so it includes updated research on proper biocompatibility testing procedures.
Though ISO 10993 contains 20 sections, only a handful are relevant to cervical implant manufacturers. Among them are sections on cytotoxicity, sensitization and systemic toxicity testing. The ISO 10993 also details how manufacturers are to sample their implants for testing. During testing, the test sample must be identical to a final version of the implant. In other words, the test sample must be converted, processed, sterilized and packaged using the same methods as the finished implant.
The testing procedures on permanent implants, like a cervical cage, are the most rigorous. Further, ISO 10993 derives its testing recommendations from respected standards and organizations, so they are the best procedures available to researchers.
During USP Class VI testing, the biomaterial is tested in animal tissues, including tissues that the implant is expected to interface directly with. During this testing, researchers are looking for cytotoxic (harms cells), genotoxic (harms the cells’ genetic material) or immunogenic (produces an allergic reaction) properties. PEEK is one of the few materials to earn excellent marks during USP Class VI testing, and it has been used with confidence in thousands of patients since 1999.
4. Processability – Like other polymers, PEEK gives device manufacturers a lot of options to work with. In most cases, PEEK is machined using precise CAM and CAD processes, and because it is a high-performance polymer, PEEK withstands machining with no loss of material properties. This takes a skilled PEEK processor, though, because something as subtle as improper fiber orientation may compromise the material.
In the hands of an experienced processor PEEK can be machined to extremely tight tolerances, even when manufactured with complex shapes. PEEK also can be extruded as well into long lengths of stock shapes (rods) and medical tubing, which is particularly useful in cardiovascular applications.
Regarding cervical cages, PEEK’s processability ensures it can be machined to fit the patient’s anatomy to precision. Manufacturers are creating implants that can be sized with no loss of function.
5. Emerging potential – PEEK has only had 20 years to make an impact on medicine, so there is still plenty of research to be done on the polymer. Every new generation of PEEK implant brings better results and additional features. For example, PEEK cervical cages are now designed with materials that enhance bone-in growth and produce better integration between implant and native tissue. Hydroxyapatite and zeolite are two such materials, and many new implants are also manufactured with microporous designs that encourage bone to grow into the cage.
Research is underway on PEEK implants manufactured with titanium coatings and other surface modifications. These could provide additional solutions for better osseointegration.
PEEK cervical cages are a frontline choice in several spinal fusion procedures, and with 20 years of successful use in patients, it’s a well-deserved position. The future of PEEK implants is just as bright as the present, with advanced interbody fusion cage designs demonstrating even better osseointegration and patient outcomes.